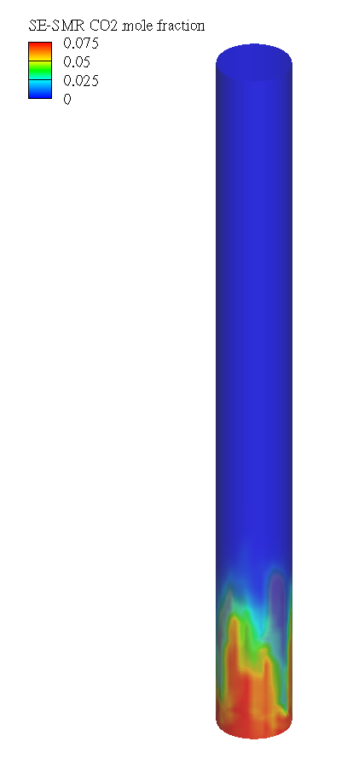
Application Model Overview
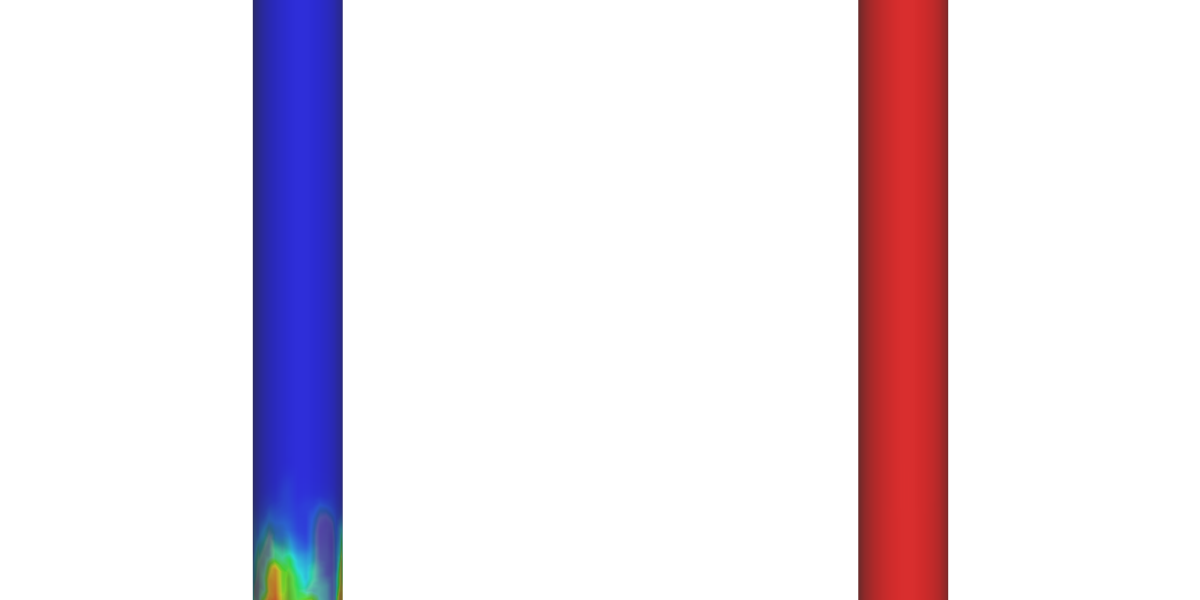
Steam methane reforming (SMR) is a leading method of hydrogen (H2) production, but it typically releases large amounts of CO2. Sorbent-enhanced SMR (SE-SMR) integrates carbon capture directly into the reforming process, reducing greenhouse gas emissions by using a solid sorbent to absorb CO2.
In this application model, Barracuda Virtual Reactor is used to simulate SE-SMR in a bubbling fluidized bed reactor, providing an accurate representation of how H2 is produced while CO2 is absorbed. By comparing SE-SMR to traditional SMR, Barracuda shows how sorbent-based CO2 capture can make hydrogen production more sustainable.
With features like compressible isothermal flow and integrated chemistry for gas-solid reactions, Barracuda helps industries model complex reactor processes, optimize operations, and reduce emissions, supporting the global shift toward cleaner energy solutions.
Additional Resources
- View reaction kinetics
- Model download and instructions
- Post-processing instructions
- Xu, Jianguo and Gilbert F. Froment. “Methane steam reforming, methanation and water‐gas shift: I. Intrinsic kinetics.” Aiche Journal.
- Wan, Z., Yang, S., Bao, G., Hu, J., Wang, H.. “Multiphase particle-in-cell simulation study of sorption enhanced steam methane reforming process in a bubbling fluidized bed reactor.” Chemical Engineering Journal.
- Xiu, G., Li, P., Rodrigues, A.. “Sorption-Enhanced Reaction Process With Reactive Regeneration.” Chemical Engineering Science.
- Sun, P., Grace, J., Lim, J., Anthony, E.. “Determination of intrinsic rate constants of the CaO–CO2 reaction.” Chemical Engineering Science.